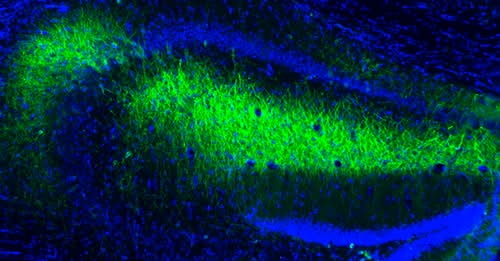
The FDA (U.S. Food and Drug Administration) has just approved Kygevvi, the first treatment for thymidine kinase 2 deficiency (TK2d), an ultra-rare mitochondrial disease causing muscle weakness, respiratory problems, and significant daily challenges.
This approval marks a historic and hopeful turning point for patients and their families, after years of research and perseverance.
Dr. Philip Yeske (UMDF) emphasizes: “Every success like this draws more attention and strengthens the hope that one day every mitochondrial disease will benefit from a treatment.”
Following Forzinity, approved in September for Barth syndrome, Kygevvi becomes the second mitochondrial treatment validated by the FDA this year, a year full of hope for the entire community!
We hope that the FDA’s decision will pave the way for the approval of the European Medicines Agency and other regulatory bodies worldwide.
To read the full article ![]()